Visible light disinfection is a lighting industry innovation wherein violet light is claimed to inactivate pathogens such as bacteria and viruses. Unlike germicidal lamps emitting ultraviolet-C radiation, visible light does not pose the considerable risks of photokeratitis (“snow blindness”) and erythema (“sunburn”) for room occupants. With the availability of inexpensive and efficient violet LEDs, the technology is being adopted by major luminaire manufacturers for everything from hospital operating theatres to residential kitchens.
As is often the case with new and exciting technologies however, the marketing efforts of both large and small manufacturers sometimes exceed the bounds of scientific evidence. For example, one manufacturer is currently claiming that its products are “… scientifically proven to kill 94% of SARS-CoV-2 under a range of conditions,” without any reference to the academic literature in support of this claim.
Other manufacturers are referencing peer-reviewed medical literature in support of their product claims, but the details can be frustratingly vague. For example, Murrell et al. (2019) reported a study of the efficacy of visible light disinfection in operating theatres wherein the manufacturer of the luminaires “… provided technical assistance before and during installation to ensure that proper illumination and disinfecting dose was achieved across the entire OR.” Unfortunately, the authors provided no indication of what the disinfecting dose (irradiance times exposure period) was.
From an engineering perspective, this is more than frustrating; it is irresponsible. If a professional lighting designer were to be asked to design or specify a visible light disinfection system in any setting, it would be the designer’s responsibility to understand both the benefits and risks of the technology, and to ensure that the target irradiance levels and operating conditions are appropriate. This presents a problem in that there are as yet no lighting or healthcare industry standards, or even guidelines, for designers to follow. (This may be contrasted with ongoing work by the ASHRAE, IEC, IES, IUVA, and other organizations to develop ultraviolet radiation standards and recommended practices.)
Ancient and Modern History
Despite appearances, visible light disinfection is by no means a new technology. The ancient Egyptians reported the health benefits of sun exposure some six millennia ago, while ancient Greek, Roman and Arabic cultures similarly recognized the therapeutic values of sunlight (e.g., Aldahan 2016). The benefits of phototherapy (or more specifically heliotherapy) were given scientific support by Downes and Blunt (1877), who reported that bacteria were inactivated by sunlight, and that violet-blue light was the most effective.
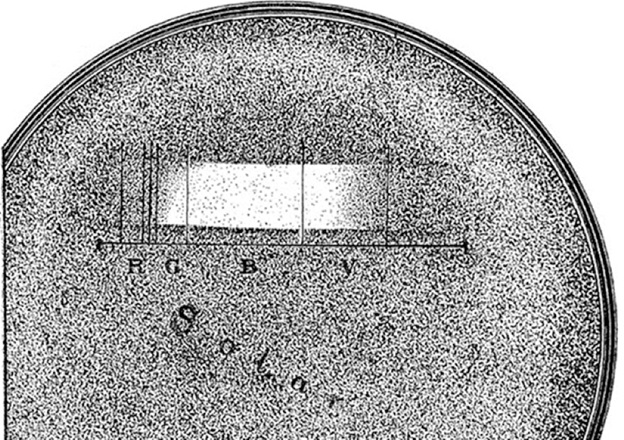
Ward (1894) quantified this antibacterial effect by dispersing sunlight with a prism and projecting it onto an agar plate with anthrax bacteria (Bacillus anthracis) colonies. The inhibition of colonies (FIG. 1) clearly demonstrated the action spectrum of sunlight, showing that blue light (B) was the most effective.
The work of Downes and Blunt inspired Niels Finsen, a Danish medical researcher, to investigate further. After initial experiments in the early 1890s with natural light, he developed an apparatus using electric carbon arcs that would later become known as the “Finsen light” (Grzybowsky and Pietzrak 2012).

Finsen began his experiments using common glass lenses to focus the electric arc emission, but knew from the work of Ward (1894) and others that ultraviolet radiation was germicidal, and so he replaced these lenses with fused quartz. However, as reported by Møller et al. (2005), Finsen used methylthioninium chloride (“methylene blue”) in solution as a heat-absorbing filter. Likely unknown to Finsen, this dye absorbs ultraviolet radiation with wavelengths shorter than 340 nm. The Finsen light therefore produced ultraviolet-A radiation and visible light, but no germicidal ultraviolet-B radiation.
Regardless, use of the Finsen light as a treatment for lupus vulgaris (a painful skin infection caused by Mycobacterium tuberculosis) was an astounding success. Between 1886 and 1901, the Finsen Institute treated 804 patients, of whom 83 percent were cured (FIG. 3). For this work, Finsen received the 1903 Nobel Prize in Medicine and Physiology. The science of phototherapy was (re)borne.
How It Works
Germicidal lamps, including low-pressure mercury vapour arc lamps emitting 254-nm UV-C radiation, Kr-Cl* excimer lamps emitting 222-nm UV-C radiation, and LEDs emitting narrowband UV-B or UV-C radiation, work by disrupting the DNA of bacteria and fungal spores, and the DNA or RNA of viruses. This disables the cellular mechanisms of bacteria and fungal spores, thereby inactivating them, and prevents viruses from being able to replicate.
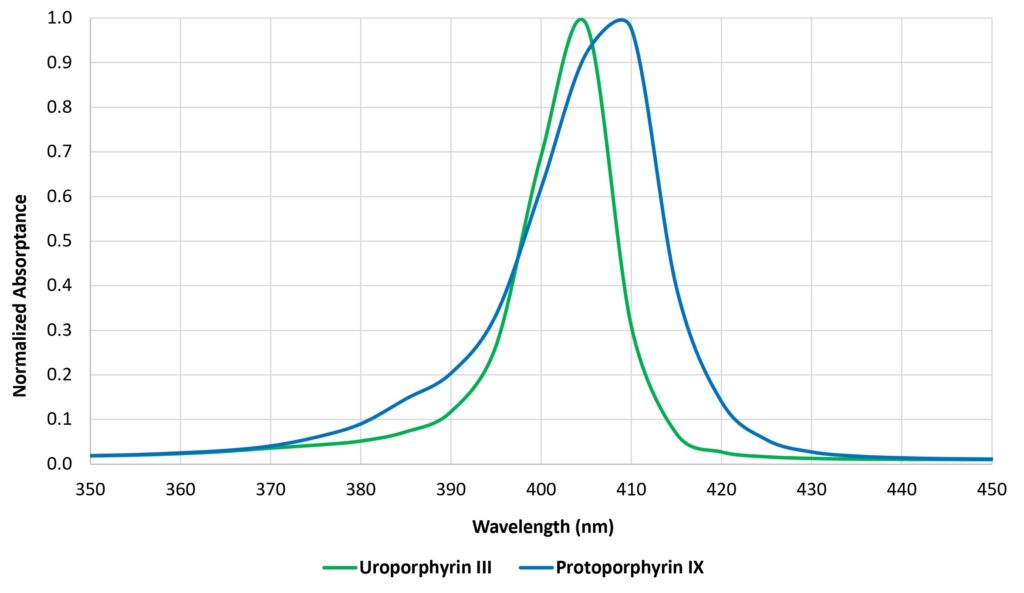
Visible light is different in that the individual photons do not have sufficient energy to disrupt DNA or RNA. Bacteria, fungi, and protozoa contain intracellular (endogenous) porphyrins that strongly absorb visible light in a region of the spectrum called the Soret band (e.g., Figure 4). These photosensitizers consequently transfer the photon’s energy to an oxygen molecule within the cell, producing a “reactive oxygen species” (ROS) molecule, such as singlet oxygen or hydrogen peroxide, that is highly reactive and cytotoxic (e.g., Kumar et al. 2015, Ramakrishnan et al. 2009). It is the ROS that inactivates the cell by disrupting its cellular machinery (e.g., Plavskii et al. 2014).
It must be said however that this is the prevailing theory; there is evidence for other explanations of how visible light inactivates pathogens, including different photosensitizers such as riboflavin acting at 450 nm (e.g., Hönes et al. 2018, Tomb et al. 2018) and different cellular inactivation mechanisms (e.g., Tomb et al. 2018, Halstead et al. 2019).
Viruses are different in that, unlike bacteria, fungi, and protozoa, they do not contain porphyrins and so in theory should not be susceptible to blue light. Nevertheless, there is growing evidence that they may be. This will be discussed below under “Viruses and Bacteriophages.”
The germicidal effects of visible light have been demonstrated for wavelengths from approximately 380 nm to 740 nm, but peak efficacy has been demonstrated at approximately 405 nm (e.g., Maclean et al. 2008, Hessling et al. 2017, Tomb et al. 2018). This is therefore the nominal wavelength employed by commercial visible light disinfection products (e.g., Rutula et al. 2018).
Various photosensitive drugs (exogeneous photosensitizers) are used in photodynamic therapy, wherein the drugs are absorbed by the diseased cells and then activated by exposure to visible light. The resultant ROS then elicit cell inactivation in the diseased tissue. Typical applications include treatment of acne, macular degeneration, psoriasis, atherosclerosis, and malignant cancers (e.g., Hamblin and Hasan 2004). This topic is relevant to visible light disinfection in that the US Federal Drug Administration may choose to regulate the use of 405 nm light as a potential photosensitizer, particularly in hospitals where patients may be on drugs with known photosensitizing effects.
Visible light disinfection, then, has a firm scientific basis for medical applications. However, its usefulness in disinfecting enclosed architectural spaces, from hospital operating theaters to residential kitchens, is considerably more nuanced. The remainder of this article therefore focuses on the many factors that must be considered when evaluating, or designing with, visible light disinfection products for various applications.
Irradiance and Dose
Knowing how visible light disinfection works does not answer the question: what irradiance and dose of visible light are needed to achieve satisfactory results? It is difficult to find relevant information, even in the many medical papers that have been published over the past fifteen years or so.
The first step is to define our terms of measurement. Irradiance, measured in milliwatts per square centimeter (mW/cm2), is the radiant equivalent of illuminance (which can be measured in lumens per square centimeter). Dose may be a less familiar concept to lighting designers, but it is the irradiance multiplied by the exposure time in seconds, expressed in joules (i.e., watt-seconds) per square centimeter (J/cm2). It is the radiant equivalent of luminous energy, but we never have a need to consider this unit in lighting design.
The next step is to understand what it means to “inactivate” pathogens in a practical sense. When exposed to constant irradiance, whether it is visible light or ultraviolet radiation, the rate of inactivation is initially exponential. That is, if 90 percent of the pathogens are inactivated after (say) one hour, 90 percent of the surviving pathogens will be inactivated after another hour. Thus, there will be 90 percent inactivation after one hour, 99 percent after two hours, 99.9 percent after three hours, and so on.
It is often more convenient to express these numbers in log10 (“log-ten”) units, where one log10 unit represents 90 percent inactivation of the initial number of pathogens, two log10 units represents 99 percent, three log10 units 99.9 percent, and so on. (Another term for log10 units is “D-value.”)
The desired degree of disinfection will depend on the application. For example, the US Food and Drug Administration (FDA) requires sterilized surgical instruments to have D-values of six or greater (99.9999 percent inactivation), on the assumption that the remaining pathogens are too few in number to cause infections. For visible light disinfection however, it is common to specify 90 percent, or one log10, inactivation.
Reducing the number of pathogens by 90 percent may not seem particularly useful, but it must be remembered that visible light disinfection, like ultraviolet-C disinfection in a hospital, should always be considered as a supplement to terminal cleaning. Its primary purpose is not to eradicate existing bacterial colonies, but basically to prevent those colonies from growing before the next terminal cleaning.
Species and Strains
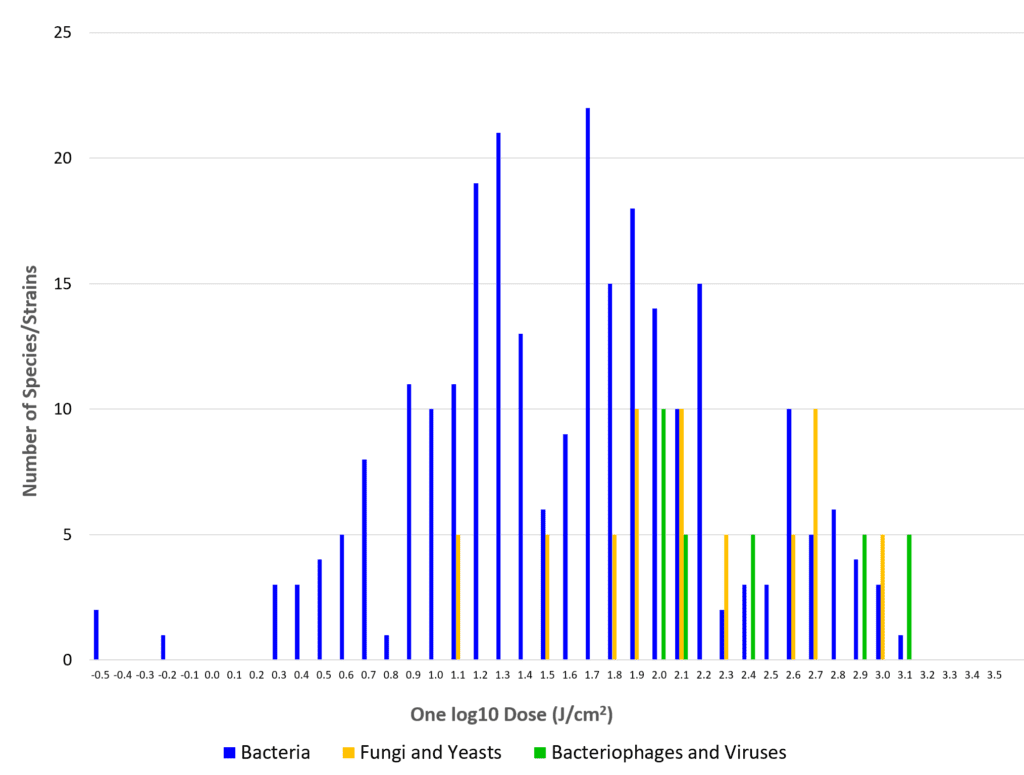
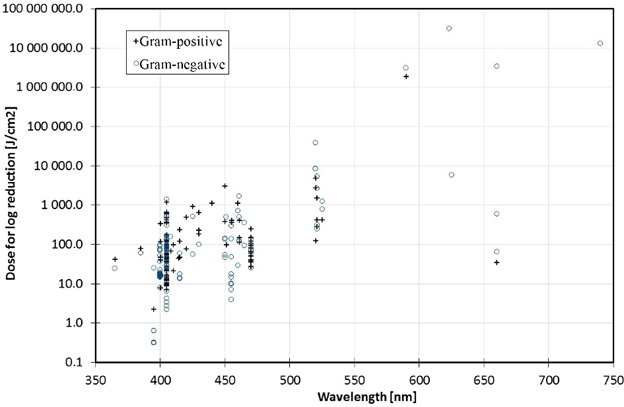
Some luminaire manufacturers list numerous pathogens that are susceptible to their products, with names like Staphylococcus aureus, Listeria monocytogenes, Salmonella enteritidis … the list goes on. Fewer than one hundred bacteria species have been examined for visible light susceptibility, and even fewer fungi and viruses. Even among this select group of common pathogens, however, the range of visible light doses needed to achieve 90 percent inactivation can be enormous (Fig. 5). For example, different strains (i.e., genetic variants) of methicillin-resistant Staphylococcus aureus (MRSA) may need between 13.7 and 1200 J/cm, depending on the laboratory conditions(e.g., Tomb et. al. 2018). In general, observed dose differences for single species of up to one order of magnitude may be expected from different laboratory studies, with fungi and viruses requiring considerably higher doses than most bacteria (Hessling et al. 2017, Tomb et al. 2018).
For test purposes, pathogens can be grown as colonies on growth media in Petri dishes or in solution, or nebulized (i.e., aerosolized) for airborne species. Their susceptibility to visible light may depend on the irradiance, the exposure time, the relative humidity, the growth medium, oxygen concentration, density (expressed in colony-forming units per milliliter, or CFU/ml), and the pathogen strain.
It gets worse: when a paper reports a given irradiance, it could be due to a single LED with a range of 10:1 over the irradiated area. For LED arrays, the irradiance could be measured at the sample surface or the LED diffuser plate (Dai et al. 2012). The transparent Petri dishes could be placed on a reflective base, which could increase and possibly double the irradiance the pathogens receive due to reflected light (Bumah et al. 2013, Hessling et al. 2017). Growth medium additives can include phenol red, which strongly absorbs in the blue/violet region of the spectrum (Hessling et al. 2022a). This makes it difficult to compare studies of minimum dose needed to achieve one log10 inactivation.
This problem is not limited to visible light disinfection. Freeman et al. (2022) discussed the issues involved in coronavirus disinfection using UV-C, noting that recommended doses ranged from 0.6 to 11,754 J/cm2. Quoting from their paper, “It highlights a substantial need to standardize a testing platform in order to evaluate germicidal inactivation doses while at the same time to understand how environmental factors influence the inactivation doses for a given pathogen.”
Biofilms
To further complicate matters, bacteria may form biofilms on surfaces to protect themselves from desiccation, antibiotics, the host body’s immune system, and other environmental factors. Biofilms are prevalent in nature, including hospitals and residences. The films themselves are not alive, but they may protect the bacteria from being inactivated by ultraviolet radiation, particularly 222-nm UV-C. (One of the arguments in favor of far-UV is that the short wavelengths are unable to significantly penetrate corneal and skin cells to cause DNA damage. The same argument presumably applies to organic biofilms.) However, McKenzie et al. (2013), Halstead, (2019b), and Huang et al. (2022) report that 405nm visible light is an effective treatment for some bacteria species encapsulated in biofilms.
Blue Light Resistance
In any microbial population, there will be a tiny fraction that are unusually resistant to UV radiation. Once 99 percent of the population has been inactivated, it is not uncommon for the rate of inactivation to decrease significantly (e.g., Enwemeka et al. 2008, Kowalski 2009). In other words, the surviving pathogens requires higher doses to inactivate them. (The same principle applies to bacteria developing resistance due to the overuse of antibiotic drugs.)
Fortunately, visible light disinfection by design does not generally seek to eradicate microbial populations, but only to limit their growth, or at best achieve 90 percent inactivation. It is therefore unlikely that pathogens will develop blue light resistance, as there is little evolutionary pressure to do so. Indeed, studies with sublethal doses of blue light (e.g., Amin et al. 2016, Tomb et al. 2017a, Leanse et al. 2018) have shown that the bacteria do not develop blue light resistance. However, Guffey et al. (2013) reported a counter-example with Staphylococcus aureus.
Ambient White Light
One issue of concern is that laboratory studies typically isolate the pathogens being studied from environmental factors, including ambient lighting where white light illumination may actively encourage bacterial and fungal colony growth (e.g., Lipovsky et al. 2009). The question is, how should such studies be interpreted in real-world conditions where pathogens are exposed to both violet light for disinfection and ambient white light for illumination? (This is discussed below under “Hospital Settings.”)
Some manufacturers are promoting visible light disinfection at irradiance levels where the exposure time is eight hours or more to achieve 90 percent inactivation in the laboratory. If the ambient illumination promotes colony growth, this may reduce the effectiveness of the visible light disinfection.
Hessling et al. (2022b) offer a further data point: using 5600K white light LEDs (which have a very significant peak at approximately 450 nm due to the blue pump LEDs), it required an illuminance of 2,400 lux over 34 hours to achieve a 90 percent reduction of Staphylococcus sp. bacteria on glazed stoneware. Adding 2.5 mW/cm2 of 405 nm irradiation reduced this to 3.5 hours, but the visual appearance of the illumination was well off the Planckian locus and decidedly purplish in color.
Viruses and Bacteriophages
Whether viruses and bacteriophages (viruses that infect bacteria) are susceptible to visible light is a topic of ongoing research. It has been argued that they should not be because they do not contain porphyrins or other endogenous photosensitizers that are capable of generating ROS. However, viruses examined in the laboratory may be suspended in organically-rich growth media such as fetal bovine serum, which can form ROS (Tomb et al. 2017b, De Santis et al. 2020, Stasko et al. 2021, Hessling et al. 2022a). Also, there may be endogenous photosensitizers in the host cells (Tomb et al. 2014, Rathnasinghe et al. 2021). Alternatively, the host cells may become inactivated due to upregulated production of damaging proteins by bacteriophages (Halstead et al. 2019).
On the other hand, some studies have examined viruses in phosphate buffered saline, which rules out endogenous photosensitizers (Lau et al. 2021, Rathnasinghe et al. 2021, Hessling et al. 2022a). However, these studies have been mostly limited to enveloped viruses (which include coronaviruses such as SARS-CoV-2), where the visible light is possibly damaging the lipids of the envelopes rather than the virus DNA or RNA.
There is therefore evidence that visible light is capable of inactivating viruses directly, but the reasons are still under investigation. Enwemeka et al. (2021a) and Hessling et al. (2022a) provide excellent summaries. Hessling et al. (2022a) in particular notes that riboflavin in solution may have a major impact on photoinactivation results, and that virus reduction with visible light may require considerably higher doses under other conditions, such as in air. Quoting the authors, “This is especially important in the ongoing corona[virus] pandemic, where frightened citizens seek protective measures and companies might offer deceptive security based on misunderstood studies.”
Different Wavelengths
Most visible light disinfection studies conducted to date have focused on the spectral region of 385 nm to 420 nm (Hessling et al. 2017, Tomb et al. 2018). However, bactericidal effects of visible light have been demonstrated with wavelengths of up to 740 nm, as shown in Figure 6 (Hessling et al. 2017). These are presumably due to the absorption characteristics of different endogenous photosensitizers, such as riboflavin around 450 nm (Hönes, K., et al. 2018). However, the dose required for inactivation generally increases exponentially with wavelength.
Spectral Interactions
Most of the studies to date have also focused on narrow spectral bands generated by quasimonochromatic LEDs. One study examined the combination of 405-nm blue light as an antibacterial agent and 880-nm near-infrared radiation as a means of tissue repair, but found that the 880-nm radiation by itself promoted bacterial growth (Guffey and Wilborn 2006). Another study used an array of phosphor-coated white LEDs with both 405 nm and 450 nm peak wavelengths, and with an estimated 6500 K correlated color temperature (CCT), to inactivate SARS-CoV-2 viruses (De Santis et al. 2020). However, the authors acknowledged that the growth medium for the infected cells contained ten percent fetal bovine serum, which may have contributed to the virus inactivation.
In a review titled “Light as a Broad-Spectrum Antimicrobial,” Gwynne and Gallagher (2018) noted that while Enterococcus bacteria are resistant to blue light, they are sensitive to near- and mid-infrared radiation. Also, Trichophyton rubrum fungi (responsible for the fungal infection onychomycosis) are sensitive to green light. The higher dose requirements may preclude these longer wavelengths from practical whole-room visible light disinfection, but more research is clearly required.
Pulsed Blue Light
Several in vitro studies have investigated the effect of pulsed blue light on the efficacy of visible light disinfection. Gillespie et al. (2017), for example, found that when exposing MSRA bacteria to 116 mW/cm2 of 405 nm radiation, varying the pulse width duty cycle from 25 to 100 percent had little effect on the dose required to achieve the same degree of inactivation. The pulse width modulation (PWM) frequency was varied from 100 Hz to 10 kHz, with 1000 Hz showing the best performance, and with 35 percent energy savings for a 50 percent duty factor. The authors speculated that the cell porphyrins become saturated with continuous exposure, and that the off period of each cycle enables the absorbed light to generate ROS with fewer photons being absorbed unnecessarily.
Masson-Meyers et al. (2019) similarly found that a duty cycle of 33 percent and a PWM frequency of 33 kHz was optimal for 450 nm radiation treating acne vulgaris caused by the Propionibacterium acnes bacterium. The experiments involved microLEDs mounted on flexible plastic sheets that were applied to the infected skin, but remarkably the required irradiance to achieve 100 percent eradication of the bacteria was 40 to 100 times less than previously reported results for in vitro experiments involving MSRA bacteria. However, this involved repeated treatments every four hours that were timed to coincide with the replication cycle of P. acnes, as opposed to single exposures of bacterial cultures in the laboratory. Further laboratory experiments with otherwise identical test conditions are likely required to confirm these results.
Bumah et al. (2020) demonstrated that pulsed 450 nm blue light suppresses the formation of MSRA and Propionibacterium acnes bacteria in planktonic cultures and bacterial biofilms. Irradiances of 3mW/cm2 and three doses of 7.6 J/cm2 were sufficient to completely eradicate MSRA bacteria in solution, while 2 mW/cm2 and 5 J/cm2 were sufficient to eradicate P. acnes bacteria. Assuming that “eradication” means 99.9 percent inactivation, this implies that the doses required for 90 percent inactivation would be 3.8 J/cm2 and 2.5 J/cm2 respectively.
Enwemeka et al. (2021b) demonstrated that pulsed blue with wavelengths ranging from 405 nm to 450 nm inactivated several strains of human coronavirus, but the efficacy varied by strain. Further, the antiviral effect was more pronounced at higher than lower irradiances.
Hospital Settings
Most of the above studies were in vitro experiments performed in laboratories. This is of course necessary to isolate specific species and strains, and to control or eliminate the influence of environmental conditions such as ambient light and temperature, relative humidity, growth medium, and more.
Investigating the efficacy of visible light disinfection in vivo under real-world conditions is more difficult, even in hospital settings such as operating theaters and in-patient rooms. Maclean et al. 2010 and Bache et al. 2012, for example, reported on the use of two ceiling-mounted 405 nm LED arrays in burn unit isolation rooms with a floor area of approximately 10 m2. Unfortunately, the irradiance levels “… were set, on the basis of extensive laboratory experimentation, to be sufficient to cause significant inactivation of exposed bacteria within the room environment” (Maclean et al. 2010). The remainder of the papers were sufficiently detailed, but the lack of irradiance information makes the experiments irreproducible.
Both papers noted that the installations were in compliance with the International Commission on Non-Ionizing Radiation Protection (ICNIRP) guidelines on exposure limits for incoherent visible radiation (ICNIRP 2013). These guidelines however are concerned with light source radiance leading to possible retinal injury; they do not address irradiance levels.
The authors referred to their visible light disinfection unit as a “High-Intensity Narrow-Spectrum light Environmental Decontamination System” (HINS-light EDS). This is useful in that Halstead et al. (2019) later described this device as “a ceiling-mounted LED array which delivers low-irradiance 405-nm light (irradiance, 0.1 to 0.5 mW/cm2) continuously to decontaminate surface in hospital operating theaters.”
Bache et al. (2012) reported that from over 1,000 environmental samples taken from inpatient isolation rooms and an outpatient clinic, there was a significant reduction in the average number of bacterial colonies (27 to 75 percent) following HINS-light EDS use with 14-hour daily exposures. If we assume an average of 50 percent (0.70 log10) reduction, it would require 20 hours of exposure to achieve 90 percent reduction. Further assuming an average irradiance of 0.3 mW/cm2, the dose would be 21.6 J/cm2.
The study involved Staphylococcus aureus, and referenced Maclean et al. (2008). From this earlier laboratory study, the required dose for 90 percent reduction was 9.8 J/cm2. While noting that the burn unit rooms had HEPA filters and underwent daily terminal cleaning to minimize the risk of hospital-acquired wound infections, the results were commensurate with what would be expected from laboratory dose requirements.
Maclean et al. (2013) reported on a similar study involving the use of HINS-light EDS units in occupied isolation rooms of intensive care units, but with a focus on the total bacterial contamination around the room rather than just MSRA bacteria. The units were again operated for 14 hours per day in synchrony with hospital lighting. The authors reported an average 50 percent reduction in contamination levels while the units were in use. Moreover, this included indirectly illuminated surfaces within the room, with an approximately 2:1 difference in bacterial reduction.
Bache et al. (2017) continued these studies within the burn unit isolation rooms, finding that there was no correlation between irradiances levels and bacterial inactivation over a range of 0.0023 mW/cm2 to 0.231 mW/cm2, but that there was a strong correlation between exposure time (i.e., dose) and bacterial inactivation. The authors attributed this to the bacteria being suspended in air almost indefinitely, which would expose them to higher spherical irradiance levels near the LED arrays due to air movement within the room. Related to this, Dougall et al. (2018) reported that Staphylococcus epidermidis was three to four more times susceptibility to 405-nm irradiation in aerosol form than when in liquids or on surfaces. This is similar to the susceptibility of most pathogens to UV-C radiation (e.g., Freeman et al. 2022, Kowalski 2009).
This highlights perhaps the most significant difference between ultraviolet radiation and visible light for whole-room disinfection. In most applications, UV-C radiation is used to disinfect air, so that proper air flow is a critical parameter. To date, however, visible light disinfection research has focused on surface disinfection. Studies such as Bache et al. 2017 suggest that visible light may be useful in air disinfection, but there is no conclusive evidence that this is true.
Maclean et al. (2013) further suggest that air disinfection may be involved, as the relatively modest 2:1 difference in bacterial reduction for shadowed surfaces is surprising when irradiance levels under tabletops, for example, are likely five or more times less than exposed surfaces (assuming 20 percent floor reflectance).
Murrell et al. (2019) reported on the successful use of commercial visible light disinfection luminaires in an orthopedic operating room, but did not disclose the irradiance levels. Instead, they stated that, “The manufacturer provided technical assistance before and during installation to ensure that proper illumination and disinfecting dose was achieved across the entire OR.” Again, the lack of irradiance information makes their experiments irreproducible.
Rutala et al. (2018) reported a study involving a 12.5 m2 windowless room equipped with two 61 cm ´ 61 cm (2-foot square) “blended-white light” LED luminaires and one 61 cm´ 122 cm “blue light” LED luminaire. The white light luminaires provided both disinfection radiation (presumably 405 nm) and “ambient white” light illumination for use in normal clinical applications in an occupied room with an irradiance of 0.12 mW/cm2 to 0.16mW/cm2 on the pathogen surface, while the disinfecting “blue light” luminaire provided an irradiance of 0.34 mW/cm2 to 0.44 mW/cm2.
This study is deficient in that without knowing the spectral power distribution of the luminaires, it is again difficult to reproduce the experiments. Nevertheless, the results shown in Table 1 for 90 percent reduction are informative. The four pathogens are common infectious bacteria in hospitals, but E. faecalis (responsible for urinary tract infections and meningitis) did not respond to white light, while C. difficile (responsible for a quarter of million hospital infections per year) did not respond to white or blue light at all.
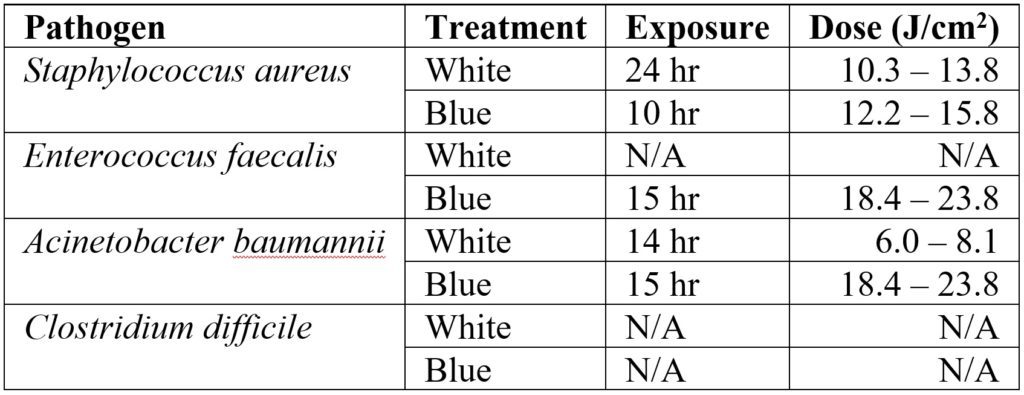
These results are crucial because they highlight an important issue with visible light disinfection; as shown in Figure 5, the range of doses required for different species and even strains of pathogens is enormous. (Note that the abscissa of Figure 5 is logarithmic.) Clostridium difficile is a spore-forming bacterium that exists in two states: vegetative and endospore. In its vegetative state, it requires a dose of 13 to 77 J/cm2 for 90 percent reduction; in its endospore state, the dose ranges from 426 J/cm2 to 736 J/cm2.
The record holder however is Aspergillus niger, a common food contaminant that also forms black mold in indoor environments. According to Murdoch et al. (2013), its spores require an astonishing 23,000 J/cm2 to achieve 90 percent reduction.
Sunlight
As with any new technology, there will be questions concerning potential health hazards. Any commercial visible light disinfection luminaire should of course satisfy the guidelines for retinal safety (ICNIRP 2013), but there are other concerns. In reviewing the safety of violet-blue light exposure, Christensen et al. (2021) reported that exposure to 400 to 500 nm blue light with a dose exceeding 65 J/cm2 can result in pigment darkening. The authors calculated that with typical indoor light sources, it would require from two weeks to 20 months to reach the threshold dose.
This is somewhat misleading, however. With the sun at zenith on a clear day, the radiant flux in the spectral region of 400 nm to 500 nm is about 14 mW/cm2(ASTM 2020). The time required to receive a 65 J/cm2 is therefore about 80 minutes. Still, the authors made a valid observation: visible light disinfection systems may pose a risk to people with photosensitive diseases, or who are taking photosensitizing drugs.
This highlights another issue with visible light disinfection. The health benefits of sunlight have been known for six millennia, and the research referenced here makes it clear why. On a clear day with the sun at zenith, the radiant flux in the Soret band (~390 nm to 420 nm) is about 5 mW/cm2 (and about 0.5 mW/cm2 on an overcast day). Over eight hours, this is about 150 J/cm2.
Simply put, direct sunlight is an excellent visible light disinfectant (not to mention the antimicrobial effects of UV-A and UV-B radiation). Schuit et al. (2020), for example, reported that the half-life (70 percent reduction) of influenza viruses in full direct sunlight is about 150 seconds.
To put this into perspective, 5 mW/cm2 translates into 500 watts of 405 nm radiant flux for a 10 m2 (108 square foot) room. If direct sunlight is available, it is a far better choice for visible light disinfection than electric lighting.
Whole-Room Disinfection
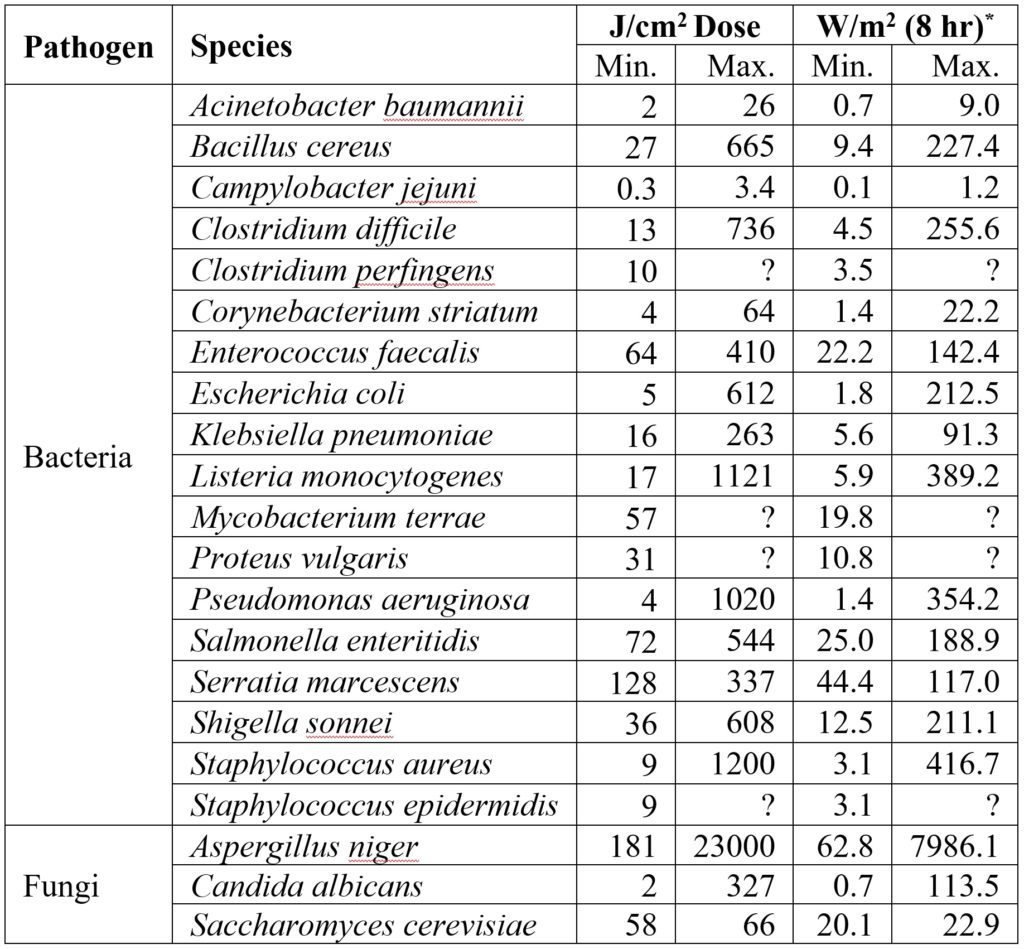
As shown by the studies cited in “Hospital Settings,” it is evident that whole-room visible light disinfection systems are useful as a supplement to terminal cleaning in hospital settings. This includes both occupied in-patient rooms with continuous illumination and unoccupied spaces (such as operating theaters) where higher blue light irradiances can be employed. Typical irradiances will be on the order of 0.1 to 0.5 mW/cm2 (one to five W/m2), with typical exposure times of 8 to 14 hours to prevent pathogens from proliferating.
It must also be understood, however, that whole-room visible light disinfection systems cannot deliver the irradiances and doses needed to control, let alone eradicate, such common hospital pathogens as Clostridium difficile, let alone the common food contaminant Aspergillus niger.
One luminaire manufacturer currently claims that its products are effective against 26 bacteria, yeast, fungi, and viral species. Referring to Tomb et al. (2018), it is evident that many of these species may require doses for 90 percent inactivation that are far in excess of what practical whole-room visible light disinfection systems can provide.
* – Based on 0.5 mW/cm2 irradiance.
As shown in Table 2, the optical power required to inactivate some pathogens can be ludicrous. Assuming a radiant efficiency of 70 percent, it would require an electrical load of 900 watts to over 100 kilowatts to control an Aspergillus niger colony in a small room!
To be fair, some of these maximum doses are related to specific laboratory conditions that may not reflect those of whole-room applications. Nevertheless, the message is clear: the marketing efforts of both large and small luminaire manufacturers must sometimes be viewed with a critical eye.
Conclusions
As professional lighting designers, we would obviously prefer recommendations such as, “the visible light irradiance on the workplane shall be between 0.1 and 0.5 J/cm2, and the daily radiant dose shall be no less than 12 hours.” Unfortunately, the reality is that we live in a dirty world with innumerable species and strains of pathogens that visible light cannot inactivate by even 90 percent without impractical irradiance levels and radiant doses.
The studies cited in this review have demonstrated that visible light has antimicrobial properties under carefully controlled laboratory conditions and (to a lesser extent) in hospital settings that were already designed to minimize the prevalence of pathogens through HEP-filtered air and daily terminal cleaning. They also however clearly demonstrate that their results cannot be extrapolated to predict the performance of visible light disinfection systems in generic whole rooms, let alone provide evidence and support for lighting design guidelines.
In summary, visible light disinfection technology may be useful in some applications, but it is by no means a panacea. When designing or specifying a disinfection system, both the lighting designer and the client will need to thoroughly understand the capabilities and limitations of the technology, and agree upon its purpose.
As for validation of the installed system … that is another topic entirely.
Acknowledgements
Thanks to Doug Steel of NeuroSense, Martin Hessling of the Institute of Medical Engineering and Mechatronics, Ulm University of Applied Sciences, and David Woodward of Signify for their thoughtful and detailed review comments.
References
Aldahan. A. S., et al. 2016. “Sun Exposure in History,” JAMA Dermatology 152(8):896. https://doi.org/10.1001/jamadermatol.2015.5660.
Amin, R. M., et al. 2016. “Antimicrobial Blue Light Inactivation of Pseudomonas aeruginosa by Photo-Excitation of Endogenous Porphyrins: In Vitro and In Vivo Studies,” Lasers in Surgery and Medicine 48(5):562-568. https://doi.org/10.1002/lsm.ss474.
ASTM. 2020. G173-03(2020), Standard Tables for Reference Solar Spectral Irradiances: Direct Normal and Hemispherical on 37° Tilted Surface. ASTM International.
Bache, S. E., et al. 2012. “Clinical Studies of the High-Intensity Narrow-Spectrum Light Environmental Decontamination System (HINS-light EDS) for Continuous Disinfection in the Burn Unit Inpatient and Outpatient Settings,” Burns 38:68-79. https://doi.org/10.1016/j.burns.2011.03.008.
Bache, S. E., et al. 2017. “Universal Decontamination of Hospital Surfaces in an Occupied Inpatient Room with a Continuous 405 nm Light Source,” J. Hospital Infection 98(1):P67-73. https://doi.org/10.1016/j.jhin.2017.07.010.
Bumah, V. V., et al. 2013. “Wavelength and Bacterial Density Influence the Bactericidal Effect of Blue Light on Methicillin-Resistant Staphylococcus aureus (MSRA),” Photomedicine and Laser Surgery 31(3):547-553. https://doi.org/10.1089/pho.2012.3461.
Bumah, V. V., et al. 2013. “Wavelength and Bacterial Density Influence the Bactericidal Effect of Blue Light on Methicillin-Resistant Staphylococcus aureus (MSRA),” Photomedicine and Laser Surgery 31(3):547-553.
Bumah, V. S., et al. 2020. “Pulsed 450 nm Blue Light Suppresses MRSA and Propionibacterium acnes in Planktonic Cultures and Bacterial Biofilms,” J. Photochemistry & Photobiology B: Biology 202:111702. https://doi.org/10.1016/j.jphotobiol.2019.111702.
Christensen, T., et al. 2021. “Violet-Blue Light Exposure of the Skin: Is There Need for Protection?”, Photochemical & Photobiological Sciences 20:615-625. https://doi.org/10.1007/s43630-021-00043-9.
Dai, T., et al. 2012. “Blue Light for Infectious Diseases: Propionibacterium acnes, Helicobacter pylori, and Beyond?”, Drug Resistance Update 15(4):223-236. https://doi.org/10.1016/j.drup.2012.07.001.
De Santis, R., et al. 2020. “Rapid Inactivation of SARS-CoV-2 with LED Irradiation of Visible Spectrum Wavelengths,” J. Photochemistry and Photobiology. https://doi.org/10.1016/j.jpap.2021.100082.
Dougall, L. R., et al. 2018. “Efficacy of Antimicrobial 405 nm Blue-Light for Inactivation of Airborne Bacteria,” Proc. SPIE 10479, 107491G. https://doi.org/10.1117/12.2289987.
Downes, A., and T. Blunt. 1877. “Research on the Effect of Light Upon Bacteria and Other Organisms,” Proc. Royal Society of London 26:488-500.
Enwemeka, C. S., et al. 2008. “Visible 405 nm SLD Light Photo-Destroys Methicillin-Resistant Staphylococcus aureus (MRSA) in vitro,” Lasers in Surgery and Medicine 40(10):734-737. https://doi.org/10.1002/lsm.20724.
Enwemeka, C. S., et al. 2020. “Light as a Potential Treatment for Pandemic Coronavirus Infections: A Perspective,” J. Photochemistry & Photobiology B: Biology 207:111891. https://doi.org/10.1016/j.jphotobiol.2020.111891.
Enwemeka, C. S., et al. 2021a. “The Role of UV and Blue Light in Photo-eradication of Microorganisms,” J. Photochemistry & Photobiology 8:100064. https://doi.org/10.1016/j.jpap.2021.100064.
Enwemeka, C. S. et al. 2021a. “Pulsed Blue Light Inactivates Two Strains of Human Coronavirus,” J. Photochemistry and Photobiology B: Biology 222:112282. https://doi.org/j.photobiol.2021.112282.
Freeman, S., et al. 2022. “Systematic Evaluating and Modeling of SARS-CoV-2 UVC Disinfection,” Scientific Reports (2022)12:5869. https://doi.org/10.1038/s41598-022-09930-2.
Gillespie, J. B., et al. 2017. “Efficacy of Pulsed 405-nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle,” Photomedicine and Laser Surgery 35(3):150-156. https://doi.org/pho.2016.4179.
Grzybowski, A., and K. Pietrzak. 2012. “From Patient to Discoverer – Niels Ryberg Finsen (1860 – 1904) – the Founder of Phototherapy in Dermatology,” Clinics in Dermatology 30:451-455. https://doi.org/10.1016/j.clindermatol.2011.11.019.
Guffey, J. S., and J. Wilborn. 2007. “In vitro Bactericidal Effects of 405-nm and 470-nm Blue Light,” Photomedicine and Laser Surgery 24(6):684-688. https://doi.org/10.1089/pho.2006.24.684.
Guffey, J. S., et a. 2013. “Evidence of Resistance Development by Staphylococcus aureus to an in vitro, Multiple Stage Application of 405 nm Light for a Supraluminous Diode Array,” Photomedicine and Laser Surgery 31(4):179-182. https://doi.org/10.1089/pho.2012.3450.
Gwynne, P. J., and M. P. Gallagher. 2018. “Light as a Broad-Spectrum Antimicrobial,” Frontiers in Microbiology 9 Article 119. https://doi.org/10.3389/fmicb.2018.00119.
Halstead, F. D., et al. 2019. “Violet-Blue Light Arrays at 405 nm Exert Enhanced Antimicrobial Activity for Disinfection of Monomicrobial Nosocomial Biofilms,” Applied and Environmental Microbiology 85(21): 1-16. https://doi.org/10.1128/AEM.01346-19.
Hamblin, M. R., and T. Hasan. 2004. “Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease?”, Photochemistry & Photobiology Sciences 3(5):436-450. https://doi.org/10.1039/b311900a.
Hessling, M., et al. 2017. “Photoinactivation of Bacteria by Endogenous Photosensitizers and Exposure to Visible Light of Different Wavelengths – A Review on Existing Data,” FEMS Microbiology Letters 367. https://doi.org/10.1093/femsle/fnw270.
Hessling, M., et al. 2022a. “Review of Virus Inactivation by Visible Light,” Photonics 9:113. https://doi.org/10.3390/photonics9020113.
Hessling, M., et al. 2022b. “Surface Disinfection with White-Violet Illumination Device,” AIMS Bioengineering 9)2):93-101. https://doi.org/10.3934/bioeng.2022008.
Hönes, K., et al. 2018. “405 nm and 450 nm Photoinactivation of Saccharomyces cerevisiae,” European Journal of Microbiology and Immunology 8(4):142-148. https://doi.org/10.1556/1886.2018.00023.
Huang, Y., et al. 2020. “Inactivation Efficacy of 405 nm LED Against Cronobacter sakazakii Biofilm,” Frontiers in Microbiology 11:610077. https://doi.org/10.3389/fmicb.2020.610077.
ICNIRP. 2013. “ICNIRP Guidelines on Limits of Exposure to Incoherent Visible and Infrared Radiation.” Health Physics 105(10:74-96. https://www.icnirp.org/cms/upload/publications/ICNIRPVisible_Infrared2013.pdf
Kowalski, W. 2009. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection. New York, NY:Springer.
Kumar, A., et al. 2015. “Kinetics of Bacterial Inactivation by 405 nm and 520 nm Light Emitting Diodes and the Role of Endogenous Coproporphyrin on Bacterial Susceptibility,” J. Photochemistry and Photobiology 149:37-44. https://doi.org/10.1016/j.jphotobiol.2015.05.005.
Lau, B., et al. 2021. “High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces”, Photonics 101(8):414. https://doi.org/10.3390/photonics8100414.
Leanse, L. G., et al. 2018. “Evaluating the Potential for Resistance Development to Antimicrobial Blue Light (at 405 nm) in Gram-Negative Bacteria: In vitro and in vivo Studies,” Frontiers in Microbiology 2018.024043. https://doi.org/10.3389/fmicb.2018.02403.
Lipovsky, A., et al. 2009. “Sensitivity of Staphylococcus aureus Strains to Broadband Visible Light,” Photochemistry and Photobiology 85:255-260. https://doi.org/10.1111/j.1751-1097.2008.00429.x.
Maclean, M., et al. 2008. “High-Intensity Narrow-Spectrum Light Inactivation and Wavelength Sensitivity of Staphylococcus aureus,” FEMS Microbiology Letters 285:227-232. https://doi.org/10.1111/j.1574-6968.2008.01233.x.
Maclean, M., et al. 2010. “Environmental Decontamination of a Hospital Isolation Room Using High-Intensity Narrow-Spectrum Light,” J. Hospital Infection 76:247-251. https://doi.org/10.1016/j.jhin.2010.07.010.
Maclean, M., et al. 2013. “Continuous Decontamination of an Intensive Care Isolation Room During Patient Occupancy Using 405 nm Light Technology,” J. Infection Prevention 14(5):176-181. https://doi.org/10.1177/1757177413483646.
Maclean, M., et al. 2014. “405 nm Light Technology for the Inactivation of Pathogens and its Potential Role for Environmental Disinfection and Infection Control,” J. Hospital Infection 88(1):1-11. https://doi.org/10.1016/j.hnin.2014.06.004.
McKenzie, K., et al. 2013. “Photoinactivation of Bacteria Attached to Glass and Acrylic Surfaces by 405 nm Light: Potential Application for Biofilm Decontamination,” Photochemistry and Photobiology 8(9):927-935. https://doi.org/10.1111/php.12077.
Møller, K. I., et al. 2005. “How Finsen’s Light Cured Lupus vulgaris,” Photodermatology, Photoimmunology & Photomedicine 21(3):118-124. https://doi.org/10.1111/j.1600-0781.2005.00159x.
Murdoch, L. E., et al. 2013. “Lethal Effects of High Intensity Violet 405-nm Light on Saccharomyces cerevisiae, Candida albicans and on Dormant and Germinating Spores of Aspergillus niger,” Fungal Biology 117(7-8):519-527. https://doi.org/10.1016/j.funbio.2013.05.004.
Murrell, L. J., et al. 2019. “Influence of a Visible-Light Continuous Environmental Disinfection System on Microbial Contamination and Surgical Site Infections in an Orthopedic Operating Room,” American J. Infection Control 47:804-810. https://doi.org/10.1016/j.ajic.2018.12.002.
Plavskii, V. Y., et al. 2018. “Porphyrins and Flavins as Endogenous Acceptors of Optical Radiation of Blue Spectral Region Determining Photoinactivation of Microbial Cells,” J. Photochemistry and Photobiology 183:172-183. https://doi.org/10.1016/j.photobiol.2018.04.021.
Ramakrishnan, P., et al. 2009. “Cytotoxic Reponses to 405-nm Light Exposure in Mammalian and Bacterial Cells: Involvement of Reactive Oxygen Species,” Toxicology In Vitro 23:54-62. https://doi.org/10.1016/j.tiv.2016.02.011.
Rathnasinghe, R., et al. 2021. “The Virucidal Effects of 405 nm Visible Light on SARS-CoV-2 and Influenza A Virus,” Scientific Reports 11(11):19470. https://doi.org/10.1038/s41598-021-97797-0.
Rutala, W. A., et al. 2018. “Antimicrobial Activity of a Continuous Visible Light Disinfection System” Infectious Control Hospital Epidemiology 39(10):1250-1258. https://doi.org/20.1.17/ice.2018.200.
Schuit, M., et al. 2020. “The Influence of Simulated Sunlight on the Inactivation of Influenza Virus in Aerosols,” J. Infectious Diseases 2020:221:372-378. https://doi.org/10.1093/infdis/jiz582.
Stasko, N., et al. 2021. “Visible Blue Light Inhibits Infection and Replication of SARS-CoV-2 at Doses that are Well-Tolerated by Human Respiratory Tissue,” Scientific Reports 11:20595. https://doi.org/10.1038/s41598-021-99917-2.
Tomb, R. M., et al. 2014. “Inactivation of Streptomyces phage F C31 by 405 nm Light: Requirements for Exogenous Photosensitizers?”, Bacteriophage 1:1-6. https://doi.org/10.4161/bact.32129.
Tomb, R. M., et al. 2017a. “Assessment of the Potential for Resistance to Antimicrobial Violet-Blue Light in Staphylococcus aureus,” Antimicrobial Resistance and Infection Control 6 Article 100. https://doi.org/10.1186/s13756-017-0261-5.
Tomb, R. M., et al. 2017b. “New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination,” Food Environmental Virology 9:159-167. https://doi.org/10.1007/s12560-016-9275-z.
Tomb, R. M., et al. 2018. “Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light, Photochemistry and Photobiology 94(3):445-458. https://doi.org/10.1111/php.12833.
Ward, H. M. 1894. “The Action of Light on Bacteria – III,” Philosophical Trans. Royal Society of London B: Biology 185:961-986.

0 Comments